技术服务
technical service
高效组织透明化服务(仅需1-2天);大体积组织高质量透明化;成像信号更强;全流程服务;
实验背景
完整组织的三维 (3D) 成像的高分辨率映射已被用作可视化神经解剖数据的可行方法。特别是,将组织内感兴趣细胞的空间位置或整体形态扩展到传统的生物和医学信息之外,从而获得整体信息。组织透明化技术与光学成像设备(如共聚焦和光片显微镜和成像程序)相结合,可以直接识别 3D 生物信息。
组织透明化,顾名思义就是让组织变得透明,通过生化试剂去除造成光散射和光吸收的组分,从而让组织达到折射率均一、光学均质性。组织标记是形态学研究中重要的研究方法之一。通过组织细胞特异性标记以及显微镜观察与统计,可以对目标蛋白、核酸、亚细胞结构等进行明确的定性、定位和定量分析等
应用方向:神经学,发育生物学,肿瘤学,细胞生物学,病理学、基因组学,免疫生物学,药物评价等;脑,脾,小肠,肾,肺,心脏,皮肤、肿瘤、类器官等多种完整器官。
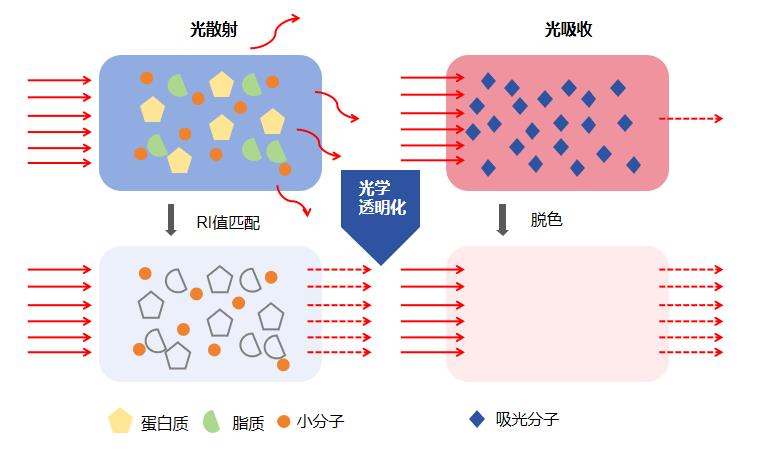
服务内容
服务模块:
为您提供实验设计,组织透明化,染色,光片/共聚焦成像与分析出图的全流程服务。以下服务模块可根据实验要求任意组合。
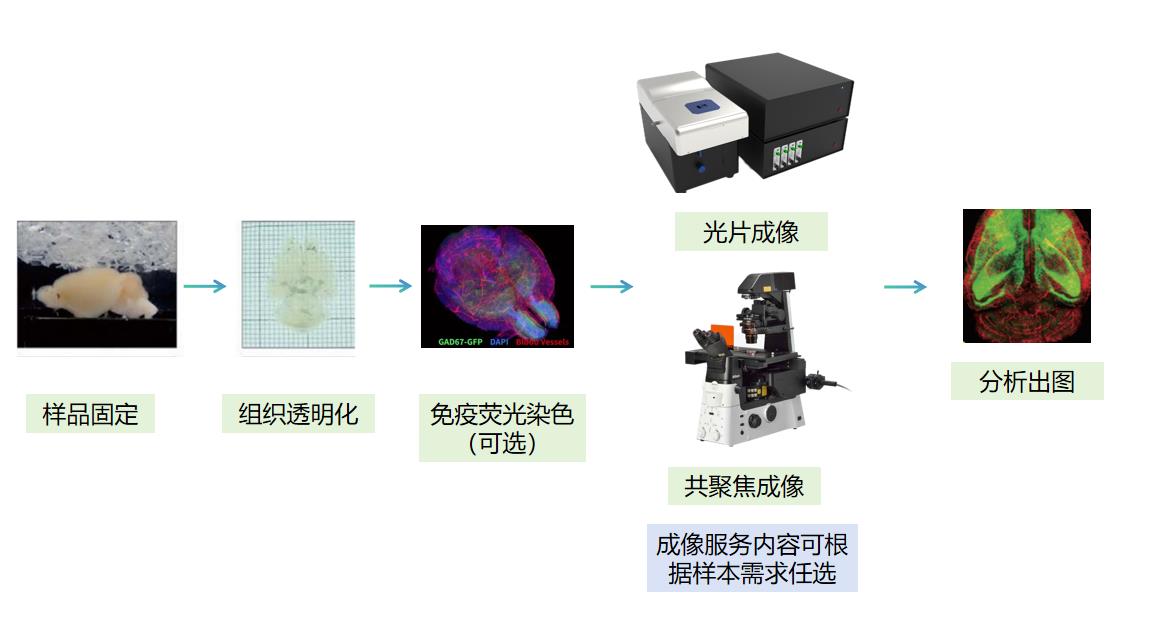
适用样本:
l 物种:斑马鱼、小鼠、兔等多种模式生物
l 组织类型:脑,脊髓,脾,小肠,肾,肺,心脏,胚胎、皮肤、肿瘤、类器官和细胞球等多种完整组织
l 标记类型:内源荧光标记样本,免疫荧光染色样本
l 样本尺寸:从大尺寸样本兔全脑到类器官球体
应用领域:
神经学,发育生物学,肿瘤学,细胞生物学,病理学、基因组学,免疫生物学,药物评价等
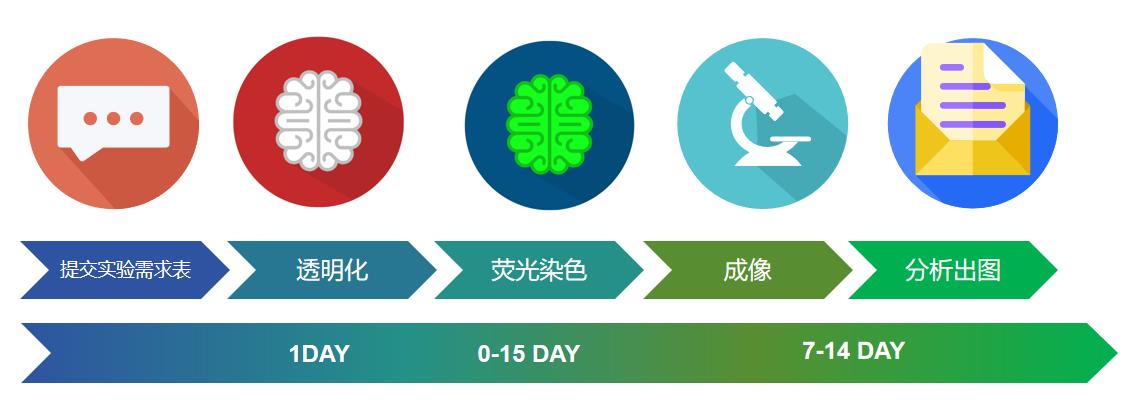
服务流程
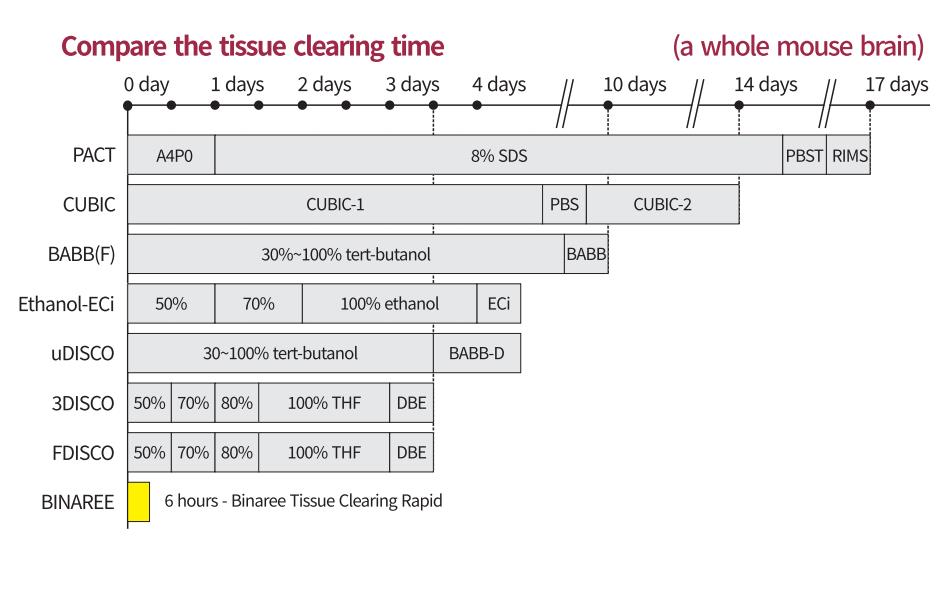
技术优势
l 组织透明化速度更快,过程更温和
l 适用各种样本类型,与大尺寸样本
l 组织透明化效果更好,样本形变更小
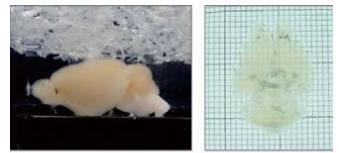
l 获得质量更好的荧光成像结果,荧光效果最长保持一个月
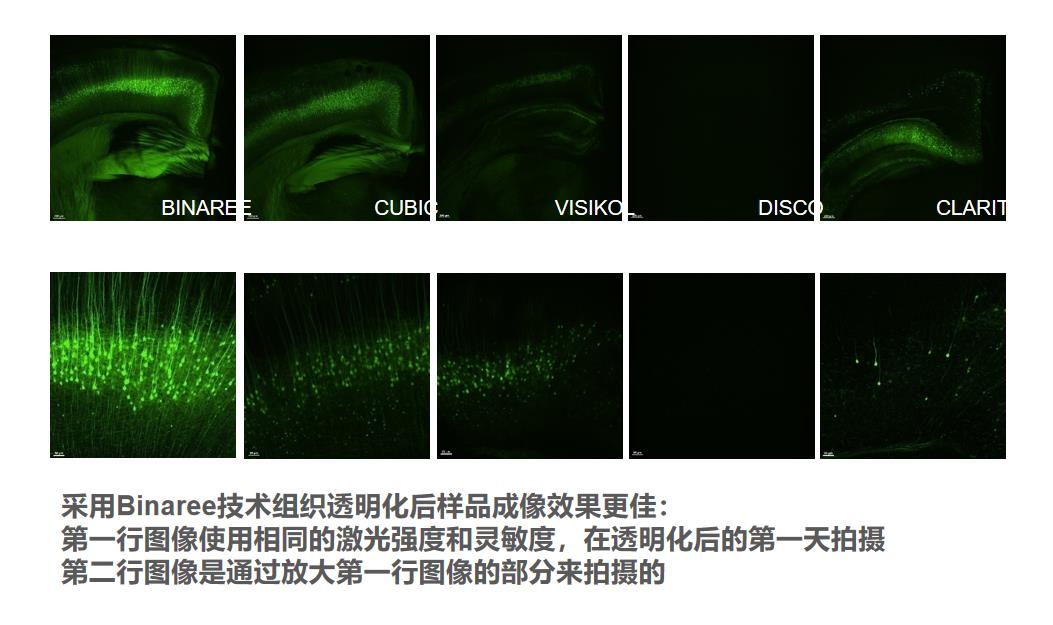
l 更优化的染色服务方案
l 全流程一站式3D组织透明化服务
服务案例
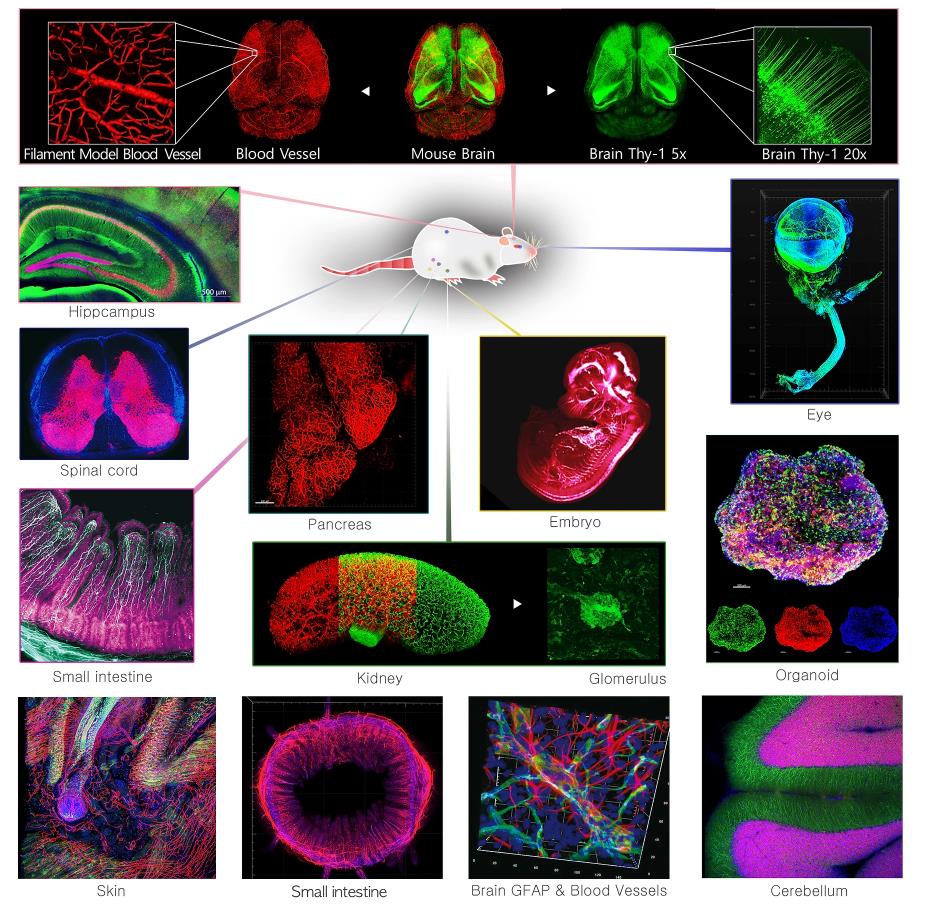
案例文献
[1] Han EC, Choi SY, Lee Y, Park JW, Hong SH, Lee HJ. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 2019;33(12):13412-13422. doi:10.1096/fj.201901575R
[2] Lee EJ, Hong SK, Choi DH, et al. Three-dimensional visualization of cerebral blood vessels and neural changes in thick ischemic rat brain slices using tissue clearing. Sci Rep. 2022;12(1):15897. Published 2022 Sep 23. doi:10.1038/s41598-022-19575-w
[3] Choi SY, Kang SH, Oh SY, et al. Differential Angiogenic Potential of 3-Dimension Spheroid of HNSCC Cells in Mouse Xenograft. Int J Mol Sci. 2021;22(15):8245. Published 2021 Jul 31. doi:10.3390/ijms22158245
[4] Kim K, Kim YG, Jung SW, et al. Three-Dimensional Visualization With Tissue Clearing Uncovers Dynamic Alterations of Renal Resident Mononuclear Phagocytes After Acute Kidney Injury. Front Immunol. 2022;13:844919. Published 2022 Mar 10. doi:10.3389/fimmu.2022.844919
[5] Mali NM, Park JM, Kim GH, Choi DH, Ramos R, Lee JH, Ku EJ, Oh JW. Deep Tissue Clearing for Three-dimensional Imaging Analysis of Murine Pancreas. Anat Biol Anthropol. 2022 Jun;35(2):57-66. doi:10.11637/aba.2022.35.2.57
[6] Lopes, M.M., Paysan, J., Rino, J. et al. A new protocol for whole-brain biodistribution analysis of AAVs by tissue clearing, light-sheet microscopy and semi-automated spatial quantification. Gene Ther 29, 665–679 (2022). doi:10.1038/s41434-022-00372-z
[7] Jeon, H., Kim, M., Park, W. et al. Upregulation of AQP4 Improves Blood–Brain Barrier Integrity and Perihematomal Edema Following Intracerebral Hemorrhage. Neurotherapeutics 18, 2692–2706 (2021). doi:10.1007/s13311-021-01126-2
[8] Heo Y, Cho WS, Maruthupandy M, Kim SK, Park JW. Biokinetics of fluorophore-conjugated polystyrene microplastics in marine mussels. J Hazard Mater. 2022;438:129471. doi:10.1016/j.jhazmat.2022.129471
[9] Kang M, Jin S, Cho H. MRI investigation of vascular remodeling for heterogeneous edema lesions in subacute ischemic stroke rat models: Correspondence between cerebral vessel structure and function. J Cereb Blood Flow Metab. 2021;41(12):3273-3287. doi:10.1177/0271678X211029197
[10] Lee S, Kim Y. Protocol for whole-brain immunostaining of the turquoise killifish after tissue clearing. STAR Protoc. 2021;2(2):100564. Published 2021 May 26. doi:10.1016/j.xpro.2021.100564
[11] Li S, Hu W, Gong S, et al. The Role of PRRC2B in Cerebral Vascular Remodeling Under Acute Hypoxia in Mice. Adv Sci (Weinh). 2023;10(25):e2300892. doi:10.1002/advs.202300892


